AATCC 168 pdf free download
AATCC 168 pdf free download.Chelation Value of Polyaminopolycarboxylic Acids and Their Salts: Copper PAN Method.
3. Terminology
3.1 chelating agent. n.—–in textile chemistry, a chemical capable of deactivating metal ions by formation of a watcr-solublc complex. SYN: sequestering agent.
4. Safety Precautions
NOTE: These safety precautions arc for information purposes only. The precautions are ancillary to the testing procedures and are not intended to be all inclusive. It is the user’s responsibility to use safe and proper techniques in handling materials in this test method. Manufacturers MUST be consulted for specific details such as material safety data sheets and other manufacturer’s recommendations. All OSIIA standards and rules must also be consulted and followed.
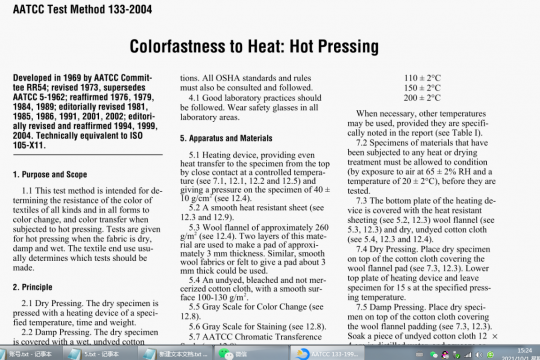
4.1 Good laboratory practices should be followed. Wear safety glasses in all laboratory areas.
4.2 All chemicals should be handled with care.
4.3 In preparing, dispensing and handling glacial acetic acid and sodium hydroxide use chemical goggles or face shield, impervious gloves and an impervious apron. Concentrated acids and bases should be handled only in an adequately ventilated laboratory hood. (‘AtJTION: Always add acid to water.
4.4 (‘upric nitrate 2.5 hydrate is corrosive to the eye and skin and is harmful if inhaled. It is an oxidi7ing material and may react with organic materials. It should be handled only in an adequately ventilated hood.
4.5 Methanol is harmful if inhaled or swallowed and is a flammable liquid. It should be stored in the laboratory only in small containers away from heat, open flames and sparks. This chemical should not be used near an open flame and should be handled only in an adequately ventilated hood.
4.6 An eyewash/safety shower should be located nearby and a self-contained breathing apparatus should be readily available for emergency use.
4.7 Exposure to chemicals used in this procedure must be controlled at or below levels set by governmental authorities (e.g.. Occupational Safety and Health Administration’s [OSI-IA] permissible exposure limits [PEL] as found in 29 CFR 1910.1000; see www.osha.gov for latest version). In addition, the American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Values (TLVs) comprised of time weighted averages (TLV-TWA), short term exposure limits (TLV-STEL) and ceiling limits (TLV-C) are recommended as a general guide for air contaminant exposure which should be met (see 14.1).
5. Reagents
5.1 Acetic acid, I .ON (CH,CO( )[I)
5.2 Cupric nitrate 2.5 hydrate (Cu(N0,), 2.51-1,0)
5.3 Ethylene diaminetetraacetic acid (free acid) (EDTA) (C10H16ON)
5.4 Methanol
5.5 1 -(2-Pyridylazo )-2-naphthol. (PAN) (C15H1 ON,)
5.6 Sodium acetate trihydrate
(CH;C(X)Na 3H,O)
5.7 Sodium hydroxide. I .O1V(NaOH)
6. Sampling
6.1 Conduct the test in triplicate; i.e., analy7c 3 test specimens from a sample.
7. Specimens
7.1 Do not use aluminum or metallic weighing pans.
7.2 For analysis of solid EDTA. HEDTA. and DTPA (either free acid or salt forms) weigh a 0.24-0.26 g specimen of the dried chelating agent to an accuracy of0.Ol g.
7.3 For analysis of commercial solution, of salts of the chelating agents. weigh a 0.49-0.51 g specimen of the solution to an accuracy of 0.01 g.
8. Conditioning
8.1 If solid chelating agent is being evaluated, dry a 2-g sample at the appropriate temperature for at least 2 h and cool in a desiccator before weighing specimens.
8.2 Dry free acid forms of chelating agents at 120°C.
8.3 Dry salt forms of chelating agents at 80°C.
9. Preparation of Reagents
9.1 Cupric Nitrate Solution: Dissolve
23.30 g of cupric nitrate 2.5 hydrate and dilute to 1.000 L in a volumetric flask.AATCC 168 pdf download.